Pure calcium carbonate (CaCO₃) is inherently white or colorless and does not appear yellow. If the calcium carbonate you observe is yellow, this is typically due to one or more of the following external factors:
1. Presence of Metallic Impurities (e.g., Iron)
Naturally sourced calcium carbonate (e.g., from limestone, marble powder, or shell powder) often contains trace amounts of iron oxides such as Fe₂O₃ or FeOOH, which are yellow, brown, or reddish-brown in color.
Even very low concentrations (e.g., tens of ppm) can impart a faint yellow tint, especially in finely ground powders.
🌰 Example: Hematite or limonite from soil or ore may be mixed into the calcium carbonate during mining or processing.
2. Organic Contamination
Calcium carbonate derived from biological sources (e.g., seashells or eggshells) may retain traces of proteins, lipids, or natural pigments.
During processing or storage under warm, humid conditions, microbial growth or oxidation of organic residues can produce yellow-colored compounds (e.g., melanoidins or humic acids).
3. Contamination During Manufacturing or Handling
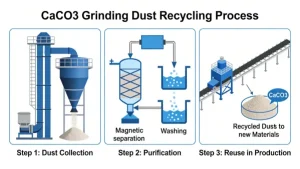
Industrial-grade calcium carbonate may come into contact with iron-containing machinery, lubricants, or oils during grinding, drying, or packaging, introducing discoloring impurities.
Some manufacturers apply surface treatment agents (e.g., stearic acid or silane coupling agents) to improve flow or dispersion; poor-quality additives or incomplete purification can lead to yellowish byproducts.
4. Photo-oxidation orAging
Although calcium carbonate itself is chemically stable, trace organic impurities may undergo photo-oxidation when exposed toUVlight or air over time, resulting in yellowing—similar to the “yellowing” seen in aged plastics.
5. Reaction with Moisture or Acidic Gases
In humid environments containing pollutants like SO₂ or NOₓ, the surface of calcium carbonate may slowly react to form calcium sulfate or nitrate, potentially releasing trapped impurities that contribute to discoloration.
How to Assess Purity?
td {white-space:nowrap;border:0.5pt solid #dee0e3;font-size:10pt;font-style:normal;font-weight:normal;vertical-align:middle;word-break:normal;word-wrap:normal;}
| Method | Description |
| Compare with high-purity reagent | Analytical-grade (AR) or chromatographic-grade CaCO₃ is a bright white, fine powder with no odor. |
| Ignition test | Organic impurities will char (turn black) or volatilize upon strong heating; pure CaCO₃ decomposes to white calcium oxide (CaO). |
| Acid dissolution test | Adding dilute hydrochloric acid should produce vigorous CO₂ bubbling and yield a clear, colorless solution. A yellow solution indicates soluble impurities like iron ions. |
Summary
✅ Calcium carbonate is not naturally yellow.
Yellow discoloration usually stems from iron oxides, organic residues, environmental contamination, or poor manufacturing practices.
For applications in food, pharmaceuticals, cosmetics, or laboratory use, always select high-purity, food-grade, or reagent-grade calcium carbonate.