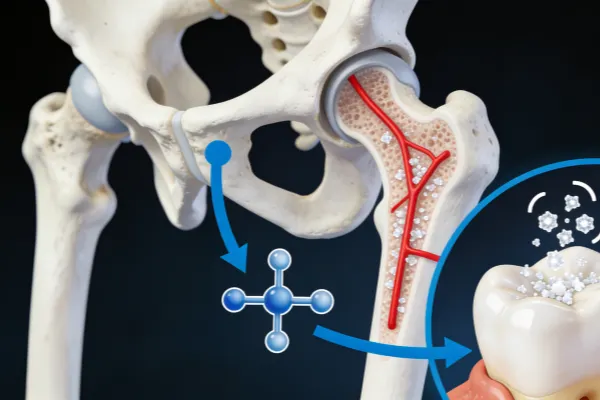
The human body does not directly require calcium carbonate (CaCO₃) as a compound; rather, it needs calcium ions (Ca²⁺). Calcium carbonate is simply one of the most common forms used to supply dietary calcium—it is a source, not a physiological necessity in itself.
Calcium is the most abundant mineral in the human body. Approximately 99% is stored in bones and teeth, while the remaining 1% circulates in blood, muscles, and extracellular fluids, where it plays several critical roles:
a) Building and Maintaining Bones and Teeth
-
Calcium combines with phosphorus to form hydroxyapatite crystals, which provide strength and rigidity to bones and teeth.
-
Inadequate calcium intake during childhood and adolescence impairs peak bone mass development.
-
In adults, chronic deficiency increases the risk of osteoporosis.
b) Muscle Contraction
-
Calcium ions trigger the sliding of muscle filaments, essential for the proper contraction of skeletal, cardiac, and smooth muscles.
-
Low blood calcium (hypocalcemia) can cause muscle cramps, spasms, or tetany (e.g., carpopedal spasm).
c) Nerve Signal Transmission
d) Blood Clotting (Coagulation)
e) Cellular Regulation
-
As a secondary messenger, calcium regulates hormone secretion, enzyme activity, cell division, and other intracellular processes.
Why Is Calcium Carbonate Commonly Used as a Supplement?
Although the body absorbs calcium as Ca²⁺ ions, it must be ingested in compound form. Calcium carbonate is widely used due to the following advantages:
| Advantage |
Explanation |
| High elemental calcium content |
Contains ~40% elemental calcium by weight—the highest among common calcium supplements—making it cost-effective. |
| Widely available and inexpensive |
Sourced from natural limestone or oyster shells; suitable for large-scale production. |
| Good stability |
Resists moisture and is easy to formulate into tablets or capsules. |
⚠️ Note: Calcium carbonate requires stomach acid (an acidic environment) to dissolve and release absorbable calcium ions. Therefore, it is best taken with meals (food stimulates gastric acid secretion). Individuals with low stomach acid (e.g., older adults or those on long-term acid-reducing medications) may absorb it poorly and might benefit more from calcium citrate, an organic form that doesn’t require acid for absorption.
Recommended Daily Calcium Intake (Based on Chinese Dietary Guidelines)
| Population Group |
Recommended Intake (mg/day) |
| Adults (18–49 years) |
800 |
| Adults ≥50 years |
1,000 |
| Pregnant or lactating women |
800–1,000 |
| Adolescents (11–17 years) |
1,000–1,200 |
Dietary sources should be prioritized, such as:
-
Dairy products (milk, yogurt, cheese)
-
Soy-based foods (tofu, dried tofu)
-
Dark green leafy vegetables (Chinese kale, amaranth)
-
Small dried fish or shrimp (note: high in sodium)
Calcium supplements like calcium carbonate may be used under medical guidance when dietary intake is insufficient.
Summary
✅ The body truly needs calcium, not calcium carbonate per se. ✅ Calcium carbonate is an efficient and economical source of calcium, but its absorption depends on adequate stomach acid. ✅ Sufficient calcium intake is vital for bone health, neuromuscular function, blood clotting, and overall cellular regulation.
Individuals with certain health conditions (e.g., kidney stones, hyperparathyroidism) should consult a healthcare provider before taking calcium supplements.