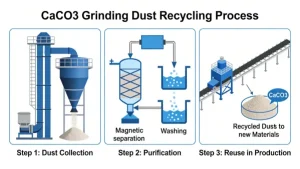
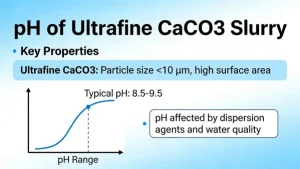
Calcium carbonate (CaCO₃) itself is a white, odorless solid that is insoluble in water. It is commonly found in limestone, marble, eggshells, and seashells. Pure calcium carbonate does not turn yellow when mixed with water. If you observe that “calcium carbonate turns yellow upon adding water,” this is usually due to one or more of the following reasons:
Impurities in the Calcium Carbonate Sample
Calcium carbonate from industrial sources, natural origins (e.g., ground limestone or shell powder), or low-grade reagents often contains impurities such as:
Iron ions (Fe²⁺/Fe³⁺): Derived from iron oxides (e.g., hematite, Fe₂O₃) in soil or ore, which can form yellow or brownish ferric hydroxide colloids in water.
Organic impurities: Such as humic substances, algal residues, or microbial metabolites that release yellow-colored compounds in water.
Other metal ions: Manganese, copper, and similar contaminants may also cause discoloration.
✅ Verification: Repeat the experiment using high-purity, analytical-grade (AR) calcium carbonate. If no yellowing occurs, the original sample likely contained impurities.
Colored or Oxidizing Substances in the Water
Tap water may contain trace amounts of chlorine, rust (iron particles), or organic matter.
Well water, river water, or unfiltered water often contains natural organic compounds like humic acid or tannins, which are pale yellow and can tint the mixture when combined with calcium carbonate.
Chemical Reactions (Less Common)
Although calcium carbonate is chemically stable, indirect reactions may occur under specific conditions:
In the presence of atmospheric pollutants like sulfur dioxide (SO₂) or nitrogen oxides (NOₓ), an acidic environment may form, partially dissolving the CaCO₃ and releasing trapped impurities.
Prolonged exposure to moisture in non-sterile environments may lead to microbial growth, causing biological discoloration.
Physical Adsorption
Calcium carbonate—especially light (precipitated) or nano-sized CaCO₃—has a large surface area and can adsorb trace pigments or colloidal particles from water, giving the suspension a faint yellow appearance.
How to Avoid or Verify?
Conduct a control test using deionized or distilled water and analytical-grade calcium carbonate.
After mixing, let the suspension settle:
If the yellow color is in the supernatant, the issue likely stems from the water.
If the precipitate itself appears yellow, the calcium carbonate contains impurities.
Filter and dry the precipitate; pure CaCO₃ should remain white when dry.
Conclusion
Yellowing upon adding water is not an inherent property of calcium carbonate—it results from sample impurities, water quality issues, or environmental factors.
Pure CaCO₃ in water should form a white suspension or precipitate, with a clear, colorless supernatant.
If you encounter this phenomenon during experiments, manufacturing, or everyday use, we recommend checking both the purity of your calcium carbonate and the quality of your water source.