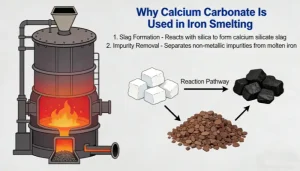
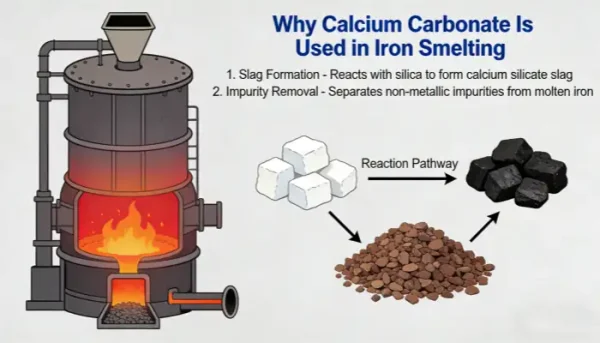
In the iron smelting process, calcium carbonate (usually added in the form of natural minerals such as limestone and dolomite, the main component of which is CaCO₃) is used. Its core role is as a flux. Through a series of reactions, it improves the performance of slag, assists in removing impurities from iron ore, optimizes the smelting process, and improves the quality of molten iron. The specific mechanism of action and significance are as follows:
1. Reduce Slag Melting Point and Improve Slag Fluidity (Core Function)
The core of iron smelting is to reduce iron oxides (such as Fe₂O₃, Fe₃O₄) in iron ore to elemental iron. However, iron ore is usually accompanied by a large amount of gangue (mainly composed of acidic oxides such as SiO₂ and Al₂O₃). These gangues have high melting points (the melting point of SiO₂ is about 1713℃, and that of Al₂O₃ is about 2072℃), while the temperature of the high-temperature zone (hearth) in the ironmaking blast furnace is about 1500℃. If they exist directly, they will form hard solids that cannot be discharged smoothly, and will also wrap the molten iron, hindering the progress of the reduction reaction.
Calcium carbonate first decomposes under the high-temperature conditions in the blast furnace: CaCO₃ → CaO + CO₂↑ (decomposition temperature is about 825℃). The generated CaO (quicklime) is a basic oxide, which can undergo a combination reaction with the acidic oxides in the gangue to form silicate/aluminate slag with a low melting point (such as CaO·SiO₂, melting point about 1250℃; 2CaO·SiO₂, melting point about 2130℃, but the melting point can drop below 1400℃ when coexisting with other components in the blast furnace). After the melting point of the slag is reduced, it is in a molten and fluid state in the blast furnace and can be smoothly discharged through the slag outlet, avoiding slagging and blockage in the furnace and ensuring the continuous progress of smelting.
2. Achieve “Slag-Iron Separation” and Improve Molten Iron Purity
The density of molten slag (about 2.8-3.0 g/cm³) is much lower than that of molten iron (about 7.0 g/cm³), so it will float on the surface of molten iron to form a “slag layer”. On the one hand, the slag can wrap and carry away unreduced impurities (such as SiO₂, Al₂O₃) in the iron ore, as well as sulfides and phosphides generated during the smelting process; on the other hand, the slag layer can cover the surface of the molten iron, isolate the air, reduce the secondary oxidation of the molten iron (avoiding the formation of ferrous oxide such as FeO, which reduces the quality of molten iron), and at the same time reduce the volatilization loss of the molten iron at high temperature. Finally, effective slag-iron separation is achieved, and molten iron with higher purity is obtained.
3. Assist in Desulfurization and Improve Molten Iron Quality
Sulfur is a harmful impurity in molten iron, which can cause “hot shortness” defects in steel products (easy to break at high temperature). Therefore, it is necessary to control the sulfur content in molten iron during iron smelting. CaO generated by the decomposition of calcium carbonate is a powerful desulfurizer, which can react with sulfides (such as FeS) in molten iron: CaO + FeS + SiO₂ → CaS + FeO·SiO₂. The generated CaS (calcium sulfide) has high stability and will dissolve in the slag and be discharged with the slag, thereby reducing the sulfur content of the molten iron. If dolomite (main component CaCO₃·MgCO₃) is used, MgO generated by decomposition can also participate in the desulfurization reaction, further improving the desulfurization effect.
4. Adjust Slag Basicity and Optimize the Smelting Reaction Environment
The “basicity” of blast furnace slag (usually expressed by the CaO/SiO₂ ratio) is a key parameter in smelting. An appropriate basicity (usually 1.0-1.2) can ensure the fluidity, desulfurization capacity and stability of the slag. CaO generated by the decomposition of calcium carbonate can accurately adjust the slag basicity: if the gangue content in the ore is high (excessive SiO₂), the slag basicity is too low, and adding calcium carbonate can supplement CaO to increase the basicity; if the basicity is too high (the slag is easy to solidify), the amount of calcium carbonate added can be adjusted to control the basicity within a reasonable range, providing a suitable chemical environment for the reduction reaction of iron ore (such as CO reducing Fe₂O₃) and improving the reduction efficiency.
5. Economic Advantage: Wide Source and Low Cost
Natural minerals of calcium carbonate (limestone, dolomite) are abundant in reserves and widely distributed. The mining and processing costs are much lower than other synthetic fluxes. During iron smelting, natural calcium carbonate minerals are directly added without complex pretreatment (only need to be crushed to an appropriate particle size), which can significantly reduce smelting costs and is the preferred flux in industrial iron smelting.
Notes:
The amount of calcium carbonate added must be strictly matched with the gangue content in the iron ore and the target slag basicity. Excessive addition will lead to excessively high slag basicity, decreased fluidity, and even increased energy consumption; if the addition amount is insufficient, the gangue cannot be completely neutralized, and problems such as high slag melting point and difficult slag discharge will still occur. Therefore, in actual production, the amount of calcium carbonate added needs to be adjusted in real time according to the composition of the iron ore to ensure stable and efficient smelting.