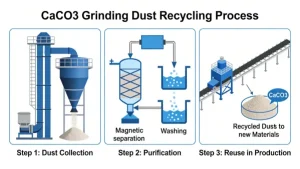
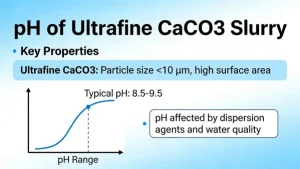
Calcium carbonate, as a versatile and cost-effective agricultural material, plays a crucial role in improving soil conditions, promoting crop growth, enhancing product quality, and ensuring the sustainability of agricultural production. Its applications in agriculture are closely linked to its physical and chemical properties such as neutralization, calcium supply, and structural regulation. The specific uses are detailed as follows:
1. Soil Acid Neutralization and Improvement of Acidic Soils
Acidic soils (with pH < 6.0) are widely distributed in many agricultural regions globally, which severely restrict crop growth by increasing the solubility of toxic heavy metals (such as aluminum, manganese), reducing the availability of essential nutrients (such as phosphorus, calcium, magnesium), and inhibiting the activity of beneficial soil microorganisms. Calcium carbonate, as a mild and efficient alkaline amendment, can neutralize soil acidity through the following chemical reactions: CaCO₃ + 2H⁺ → Ca²⁺ + CO₂↑ + H₂O. This reaction not only raises the soil pH to the optimal range for most crops (6.0-7.5) but also reduces the toxicity of heavy metals by precipitating them. Meanwhile, the neutralization process improves soil structure, reduces soil compaction, and enhances soil aeration and water permeability, creating a favorable rhizosphere environment for crop root growth.
2. Supply of Essential Calcium Nutrients for Crops
Calcium is a vital macronutrient for crop growth and development, serving as a component of cell walls (e.g., calcium pectate) and a regulator of physiological processes such as cell division, hormone signaling, and stress resistance. Calcium deficiency in crops can lead to various physiological disorders, such as blossom-end rot in tomatoes and peppers, bitter pit in apples, and tip burn in lettuce. Calcium carbonate, as a slow-release calcium fertilizer, can gradually release Ca²⁺ in the soil. Unlike water-soluble calcium fertilizers (e.g., calcium nitrate), it avoids the risk of calcium leaching in sandy soils and provides long-term and stable calcium supply for crops. This helps strengthen crop cell walls, improve lodging resistance and disease resistance, and enhance the storage and transportation stability of agricultural products.
3. Regulation of Soil Fertility and Improvement of Nutrient Availability
Calcium carbonate can indirectly improve soil fertility by regulating the availability of other nutrients. In acidic soils, phosphorus is easily fixed by aluminum and iron ions to form insoluble phosphates, making it unavailable to crops. After neutralizing soil acidity, calcium carbonate reduces the content of active aluminum and iron, promoting the conversion of fixed phosphorus into available forms (e.g., calcium phosphate) that can be absorbed by crops. Additionally, it enhances the activity of soil microorganisms (such as nitrogen-fixing bacteria and phosphate-solubilizing bacteria), which participate in the decomposition of organic matter and the cycling of nutrients (nitrogen, phosphorus, potassium), further improving the utilization efficiency of fertilizers.

4. Prevention and Control of Crop Diseases and Pests
Calcium carbonate has certain physical and chemical disease-preventive effects in agriculture. When applied as a foliar spray or soil amendment, it can form a thin protective film on the surface of crop leaves and stems, preventing the invasion of pathogenic fungi (such as powdery mildew, downy mildew) and bacteria. For example, in fruit tree cultivation, applying calcium carbonate to the soil or spraying it on the fruit surface can reduce the incidence of fruit rot. In addition, the alkaline environment created by calcium carbonate in the soil is unfavorable for the survival and reproduction of some acid-loving pests (such as root-knot nematodes), indirectly reducing pest damage.
5. Application in Organic Agriculture and Soil Remediation
In organic agriculture, calcium carbonate is recognized as a permitted soil amendment and fertilizer, as it is derived from natural minerals (e.g., limestone, calcite) and does not contain synthetic chemicals. It helps organic farms maintain soil health and improve crop yield and quality without violating organic production standards. Furthermore, in the remediation of heavy metal-contaminated soils, calcium carbonate can promote the formation of insoluble precipitates (e.g., calcium arsenate, calcium chromate) with heavy metals (arsenic, chromium), reducing their bioavailability and migration in the soil, thereby minimizing the risk of heavy metal accumulation in crops.
The application effect of calcium carbonate in agriculture is closely related to soil type, crop species, and application rate. It is necessary to test the soil pH and nutrient content first to determine the appropriate application amount and method. For example, in strongly acidic soils, a higher application rate may be required, while in neutral or alkaline soils, excessive application should be avoided to prevent soil alkalization. In addition, calcium carbonate should be applied evenly and mixed thoroughly with the soil to ensure uniform neutralization and nutrient supply. When used in combination with other fertilizers (e.g., nitrogen, phosphorus fertilizers), attention should be paid to the application time interval to avoid nutrient fixation or loss.